Properties Of Egg Proteins
The extensive use of eggs in cookery is made possible by their protein content. The protein coagulates during heating, thus bringing about thickening as in custards or the binding of pieces of food together as in croquettes. The proteins of the egg are good emulsifying agents. The proteins form elastic films when beaten, thus incorporating air, which is used as leavening in such products as angel cakes and souffles. The elasticity of the egg protein is also important in products such as popovers where the egg stretches with expansion of steam, and later coagulates to aid in forming the framework of the popover.
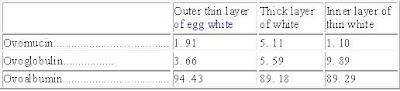
The proteins of the egg. The proteins of the white are ovoalbumin, ovoglobulin, and ovomucin. There may be small amounts of other proteins and it is also possible that each protein is made up of component fractions. Hughes and Scott give the relative proportions of the proteins in the three portions of the white as shown in Table 41.
The principal protein of the yolk is ovovitellin. Sell, Olsen, and Kremers separated salted egg yolk into a soluble lipoid fraction and an insoluble residue. The latter consisted of sodium chloride and the protein-like material of the yolk. This residue they called lecitho-protein. It composed about 32.5 per cent of the yolk. This protein fraction contained nearly one-half the total lecithin of the yolk.
Percentage Of The Total Nitrogen Contributed By Each
Of The Three Protein Fractions (Hughes and Scott)
Solubility of the proteins. The albumin of egg forms a sol with water and dilute salt solutions. The globulin forms a sol in dilute salt solutions, but not in pure water. The globulin composes about 6.5 per cent of the total proteins of the egg.
Egg-white proteins belong to the group of hydrophilic colloids. Egg white and water are mutually soluble. Usually the addition of 1 tablespoon of water to an egg white, unless it is very watery, increases its extensibility, and when the egg white is whipped a larger volume is obtained. But with increasing quantities of water a stage is reached at which the egg white loses too much of its rigidity and will no longer retain air in small bubbles, the bubbles being large and floating on the more liquid part.
The ovovitellin of the egg yolk is combined with phosphorus and belongs to the phosphoprotein group. It is insoluble in water but is soluble in dilute salt solutions and in dilute alkalies.
Isoelectric point of egg proteins. Loeb has reported the isoelectric point of egg albumin as pH 4.8. Some investigators give pH 4.7 as the isoelectric point. Above the isoelectric point the albumin combines with bases to form salts like sodium albuminate; below the isoelectric point it combines with acids to form salts like albumin acetate, citrate, or tartrate. Above the isoelectric point the protein is negatively charged; below, it is positively charged. Since the reaction of the egg white is about pH 7.6 to 9, there will probably be few combinations of egg white with alkalies or alkaline salts in food preparation that will increase its alkalinity. Many combinations are made that increase its acidity. For example, the addition of a teaspoon of cream of tartar, a salt with an acid reaction, to a cup of egg whites, proportions often used in angel food cakes, increases the acidity and lowers the pH, often to about 7.5 or 7.0. As the proportion of cream of tartar is increased, the pH is lowered to a greater extent. The addition of fruit juices and fruit pulp to egg whites to make fruit whip, souffles, or similar desserts, increases the acidity. When 1 to 2 teaspoons of lemon juice are added to an egg white the pH is lower than 4.8.
No record could be found in the literature of the isoelectric point of ovovitellin. When lemon juice is added to egg yolk, the mixture is thickest at a pH between 4 and 5, as if the greatest tendency to curdle is at this point. This might indicate that the isoelectric point of the egg yolk proteins is between pH 4 and 5. This greatest thickening occurs with about 5 cc. of lemon juice to an egg yolk.
The addition of an acid like vinegar or fruit juice to the white and yolk beaten together tends to curdle the mixture. This occurs when the acidity is in the vicinity of the isoelectric point. When sufficient acid is added to lower the pH below the isolectric point of the egg proteins, and if the salt formed, such as protein citrate, is soluble, the coagulum dissolves and the mixture becomes smooth. With the exception of salad dressings and a few sauces, there are probably not many instances in which enough acid is added to lower the pH of the food mixture below the isoelectric point of the egg protein.
Peptization of egg proteins. Peptization of egg proteins increases the tenderness of some products. Freundlich states that peptization of proteins is frequently brought about by low concentrations of electrolytes, though to accomplish this the electrolyte must be intimately mixed with the substance to be peptized. The hydroxyl, citrate, acetate, and tartrate ions are effective for peptizing egg proteins. For example, when tomato or lemon juice is added to egg in amounts to bring the pH of the egg slightly above or about the isoelectric point of egg albumin, the tenderness of omelets is definitely increased. In some instances peptization of the egg proteins is detrimental. An example of this is the thinning of salad dressings, thickened with only egg yolk, when heated above the temperature at which optimum coagulation occurs.
Sugars (sucrose, dextrose, and levulose) through peptization tend to prevent coagulation of egg protein.
Denaturation. Denaturation, by which soluble proteins are rendered insoluble, of egg proteins is brought about in a variety of ways, including the action of acids, salts, heat, mechanical agitation, and radiation. Mechanical agitation or beating of egg white, as well as the tendency of proteins in surfaces to form films, causes partial denaturation of the egg proteins. Sugar tends to prevent this denaturation.
Egg Quality (Continued)
Most of the eggs placed in low temperature storage are stored during March, April, May, and June. More than half of the annual supply of eggs is laid during these four months. Withdrawal of eggs from storage usually begins in August, reaches its peak about November, and the supply is generally exhausted by January.
Frozen eggs. The use of frozen eggs has increased very rapidly in the last 10 years. The eggs are broken out of the shell for freezing, which gives an opportunity for increasing contamination with bacteria. If eggs are frozen quickly after being broken, little bacterial growth takes place. Swenson and James found that fewer organisms survived quick freezing than delayed slow freezing. They also report that the addition of carbon dioxide to the egg batter just prior to freezing was detrimental to survival of bacteria. Eggs are frozen whole, the whites and yolks being mixed by beating. The whites and yolks are also frozen separately. Freezing alters the physical characteristics of the yolk, as it is more viscous after defrosting. Hence to prevent its becoming so stiff and gummy that it does not mix readily with other ingredients, before being frozen it is beaten and a small percentage of salt, sugar, particularly dextrose, or some suitable edible ingredient is often added. Some of these processes are patented, and the proportion of ingredients added as well as the manner of incorporation are not generally known.
Frozen whole eggs, egg yolks, and egg whites are usually prepared in 30-pound lots, though some 10-pound lots are frozen. A pound of frozen whole eggs is equivalent to about 10 fresh eggs. As yet frozen eggs are not on the retail market.
Drying. The use of dried eggs is decreasing in the United States, the frozen eggs taking their place in many products.
Oil dipping. Oil dipping, shell treating, or processing of eggs is increasing rapidly. This process consists of dipping the egg in some kind of oil to seal the pores of the egg shell, thus retaining the carbon dioxide within the egg.
Swenson, Slocum, and James report that a special white, odorless, tasteless mineral oil of right viscosity and low enough pour-point (40°F.) has been developed to be applied to the shell at temperatures of 60° to 80°F. They also state that oiling shell eggs, especially by the vacuum-carbon dioxide method, tends to maintain the pH of the egg white at 7.9 = 0.3, which is below the optimum for proteolytic activity. Sharp and Wagenen state that if eggs are oil dipped fairly soon after they are laid, the white will have a pH value of 8.0 or less at the end of the storage period. If oil dipping is to be of value in preserving the eggs they must be dipped before too much carbon dioxide has escaped.
Factors causing deterioration. The causes for deterioration of eggs may be listed as follows: (1) action of the enzyme trypsin, (2) alkaline hydrolysis, and (3) chemical changes and changes due to bacterial action. The changes brought about by these factors may be speeded up or retarded by temperature and reaction.
Enzyme action. Balls and Swenson report that trypsin, a proteolytic enzyme, is found only in the thick white. Its action, i.e., protein splitting, which reduces the proportion of thick white in the egg, is speeded up by increase in temperature, its greatest activity occurring near body temperature. Sharp has found that the weakening of the yolk membrane is greater in 2 days at 98.6°F. for eggs stored in air containing ordinary amounts of carbon dioxide and at 80 per cent humidity than after 5 days of storage at 77°, or 20 days of storage at 60.8°, or 100 days of storage at 35.6°F. Likewise a pH of about 9.25 may be reached in 2 days at 37°C, in 5 days at 16°, and in 10 days at 2°C. Or, in other words, carbon dioxide escapes more slowly and enzyme action is also slower at lower temperatures. The reaction of trypsin is also speeded up as the reaction becomes more alkaline, the optimum activity according to Swenson, Slocum, and James occurring at pH 8.4 to 8.8. Thus with loss of carbon dioxide from the egg, the action of the trypsin is increased. Balls and Swenson found that the action of the trypsin is decidedly speeded up by injecting enterokinase into the thick white, which also gives proof that the enzyme is trypsin, since enterokinase activates only trypsin. The thin white contains an anti-trypsin which inhibits the action of the trypsin. Therefore, when the thick and thin white are mixed together the action of the trypsin is inhibited. This mixing of the thick and thin white before freezing may be one reason for the claim made by many users of frozen eggs that frozen eggs are superior to and more uniform in quality than fresh eggs. Balls and Swenson state the amount of trypsin varies greatly in individual eggs of the same lot.
Alkaline hydrolysis. Alkaline hydrolysis of proteins, the breaking down of the protein into smaller units, is also speeded up as the egg becomes more alkaline by loss of carbon dioxide and as the temperature increases. Alkaline hydrolysis of protein occurs independently of enzyme action.
Chemical changes and changes due to bacterial action. Slow chemical breakdown may cause oft flavors and changes in eggs, but true rotting of eggs is caused by microorganisms.
Eggs which are infected with a large number of molds and bacteria ordinarily do not keep well. Microorganisms cannot always be detected in eggs in the shell, yet the eggs deteriorate in storage. But, in general, eggs with clean shells are comparatively free from bacteria. Reetger found that not over 4 per cent, and usually a smaller number, of eggs with clean shells were infected with bacteria. Egg shells vary in porosity and some eggs have a few very large pores. Infection with bacteria is easier through these large pores. Eggs having dirt on the shell are most easily infected. Bryant and Sharp report that washing of such eggs is not the cause for deterioration, if they are handled properly after washing. The deterioration of washed eggs is caused by bacterial infection of the egg from the dirt on the shell.
Changes in eggs with deterioration. The most important changes occurring in eggs during deterioration are: (1) the thick white becomes less viscous and jelly-like, gradually changing to a thin watery white. (2) Water passes from the white to the yolk increasing the size and fluid content of the yolk, thus decreasing the yolk solids. In addition the yolk membrane weakens and, if the weakening has progressed far enough, breaks when the shell is opened. (3) Loss of moisture usually occurs. (4) The egg may absorb foreign or off odors. (5) With continuous loss of carbon dioxide the alkalinity of the egg increases.

No comments:
Post a Comment